Abstract
Background: Allogeneic hematopoietic cell transplantation (allo-HCT) remains the only curative option for high-risk myeloid malignancies. Post-transplant cyclophosphamide (PTCY) has proven to be effective for graft versus host disease (GVHD) prophylaxis post allo-HCT in all donor settings. Given that graft-versus-tumor (GVT) effect plays a major role in reducing the risk of disease relapse, the application of PTCY must balance the risk of decreasing the GVT effect. We investigated the impact of PTCY on early mixed chimerism (EMC) after allo-HCT at our center.
Methods: 142 patients undergoing allo-HCT for myeloid malignancies between February 2015 and December 2021 were included. Six patients underwent two subsequent allo-HCTs, four patients were excluded due to early (< 30 day) transplant related mortality, resulting in a total of 144 allo-HCTs. EMC was defined as < 95% donor cells at day 90-120 post allo-HCT. Fisher's exact test was used to compare categorical variables. Univariate (UVA) and multivariate (MVA) Cox regression analyses were used to investigate factors associated with overall survival and relapse.
Results: Median age was 59 years (19-71), 41% were female and 9% were Hispanic or Latino. Majority of the patients underwent allo-HCT for acute myeloid leukemia (AML) (62%) or myelodysplastic syndrome (31%). Thirty-five percent of the patients with AML had adverse risk AML per ELN risk stratification. At time of transplant, bone marrow blast % were < 5% in 88% of patients of which 38% was MRD negative. Sixty-five percent of the patients had unrelated donors, 35% had a related donor [haplo-identical (54%) or matched sibling (46%)]. Median HCT-comorbidity index (CI) was 2 (range 0-9). Majority received peripheral blood stem cells (90%), reduced intensity conditioning (67%) and PTCY (72%) in combination with mycophenolate and tacrolimus (n=101) or sirolimus (n=2) for GVHD prophylaxis. Seventy-three percent was alive at last time of follow-up (median 2 years; range 0.15-7 years).
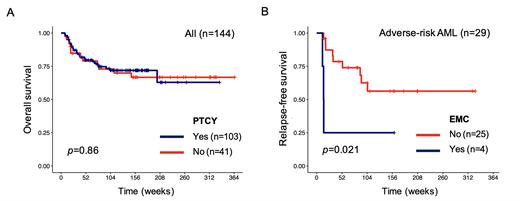
Comparing patients receiving PTCY vs no PTCY, no differences were seen in rates of acute-GVHD (p=0.26), relapse (p=0.82), or overall survival (p=0.84). A lower rate of chronic GVHD (cGVHD) (p=0.03) and a higher rate of EMC (p=0.04) were observed in patients that did receive PTCY. In UVA, MRD status (p=0.004), HCT-CI (p=0.005) and cGVHD (p=0.002) were associated with post-HCT OS, and remained independently prognostic in MVA (p=0.003,p=0.011, p=0.006, respectively). EMC (p=0.004) and AML adverse risk cytogenetics (p=0.018) were independently prognostic for relapse risk. Survival analysis restricted to adverse-risk AML patients found that EMC was associated with shorter relapse-free survival. Correlation analysis between mutational status and EMC or relapse status did not identify any mutation with a significant association, as did we not recognize any association with genomic classifications (e.g., DNA methylation or hydroxymethylation, chromatin-cohesin or splicing).
Conclusion: Our study highlights the feasibility of using PTCY in diverse donor settings. While PTCY was associated with a higher incidence of EMC and a lower rate of cGVHD, PTCY was not associated with a shorter overall survival or relapse risk, suggesting that the predictive value of EMC for relapse likely depends on the underlying disease. Further research is needed to re-evaluate the rol for PTCY in high-risk AML.
Figure 1. A. Overall survival analysis for all 144 patients stratified by PT-Cy. B. Relapse-free survival analysis for adverse-risk AML patients (n=29) stratified by EMC.
Disclosures
Patel:Agios: Membership on an entity's Board of Directors or advisory committees. Ramakrishnan Geethakumari:Kite: Consultancy; BMS: Consultancy; Rafael Pharma: Consultancy; Pharmacyclics LLC: Membership on an entity's Board of Directors or advisory committees; ADC Therapeutics: Membership on an entity's Board of Directors or advisory committees; Cellectar Biosciences: Membership on an entity's Board of Directors or advisory committees. Madanat:Sierra Oncology, Stemline Therapeutics and Novartis: Membership on an entity's Board of Directors or advisory committees; BluePrint Medicines, GERON, OncLiv: Consultancy, Honoraria.
Author notes
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal